ABOUTUS
Oryx Life Sciences is an African pharmaceutical company based in Cape Town, South Africa.
We carry a SAHPRA License in terms of Section 22 C (1)(b) of the Medicines and Related Substances Act (101 of 1965), to Manufacture, Import and Export Medicines globally. As such Oryx Life Sciences is licensed to offer the services of an Applicant (Holder of the Certificate of Registration / Marketing Authorisation Holder) to local, or non-resident dossier owners.
In addition to the above Oryx Life Sciences is also licensed to Distribute, Import and Export Medical Devices and IVDs worldwide.
Oryx Life Sciences was established in 2020 by Dr Carine Page and Dr Jaco van Zyl, two pharmaceutical scientists with a passion for Africa and its people.
Together, we leverage our regulatory, quality, scientific and clinical research expertise to effectively enable the commercialisation of healthcare products in Africa.
OUR VISION - Healthcare Product Access for Africa
OUR MISSION - To use our networks and partnerships to achieve mutual goals and product commercialisation.

Dr Carine Page
Dr Carine Page is the co-founder and Head of Regulatory- and Quality Affairs at ORYX Life Sciences.
She has 40 years’ combined experience in Clinical Research and Tertiary Education in Academia and Medical-marketing, Regulatory Affairs, Quality Assurance, Pharmacovigilance and Continuous Professional Development & Training in the Pharmaceutical Industry.
Carine has a Doctorate (PhD) in Immunology and holds a Master’s Degree in Medical Sciences. She is also a Pharmacist by profession (BPharm), with extensive experience in the Regulatory Environment in sub-Saharan Africa.
She has an in-depth knowledge of infectious diseases, auto-immune diseases and oncology and a particular interest in the research and development of new-generation biological medicines in lieu of targeted individualised treatment options for suffering patients.
Carine has a deep passion for people and for improving access to medicine to all. She has committed her career to bridging the gap between science and accessibility to life-changing medicines. Through collaboration with Healthcare Professionals and Industry Partners she continues to work tirelessly to reach this goal.

Dr Jaco van Zyl
Jaco is a South Africa based Medical Doctor, Pharmaceutical Scientist, and Health Economist with a passion for improving Healthcare access to those in need.
His areas of special interests are:
- Commercialisation of Pharmaceutical Products
- Healthcare Access
- Clinical Research and Evidence Based Medicine
- Training, Development and Skills Transfer
- Pharmacoeconomics/Health Economics
He has 25 years of work experience in the pharmaceutical industry, clinical medicine and corporate health sector.
During this time, he gained experience in the following areas:
- Strategy: General Business & Product Commercialisation Strategies
- Science: Clinical Trial Design, Development and Conduct
- Business Operations: Operational management & efficiency
- Healthcare Industry Investment: Business Analysis and Valuation
- Data Analysis: Scientific, epidemiological and health economic data
- Health Economics / Pharmacoeconomics: Demonstrating Value
- Regulation Compliance: Drug Safety & Marketing Code of Conduct
- Quality Assurance: Implementing Quality Management Systems
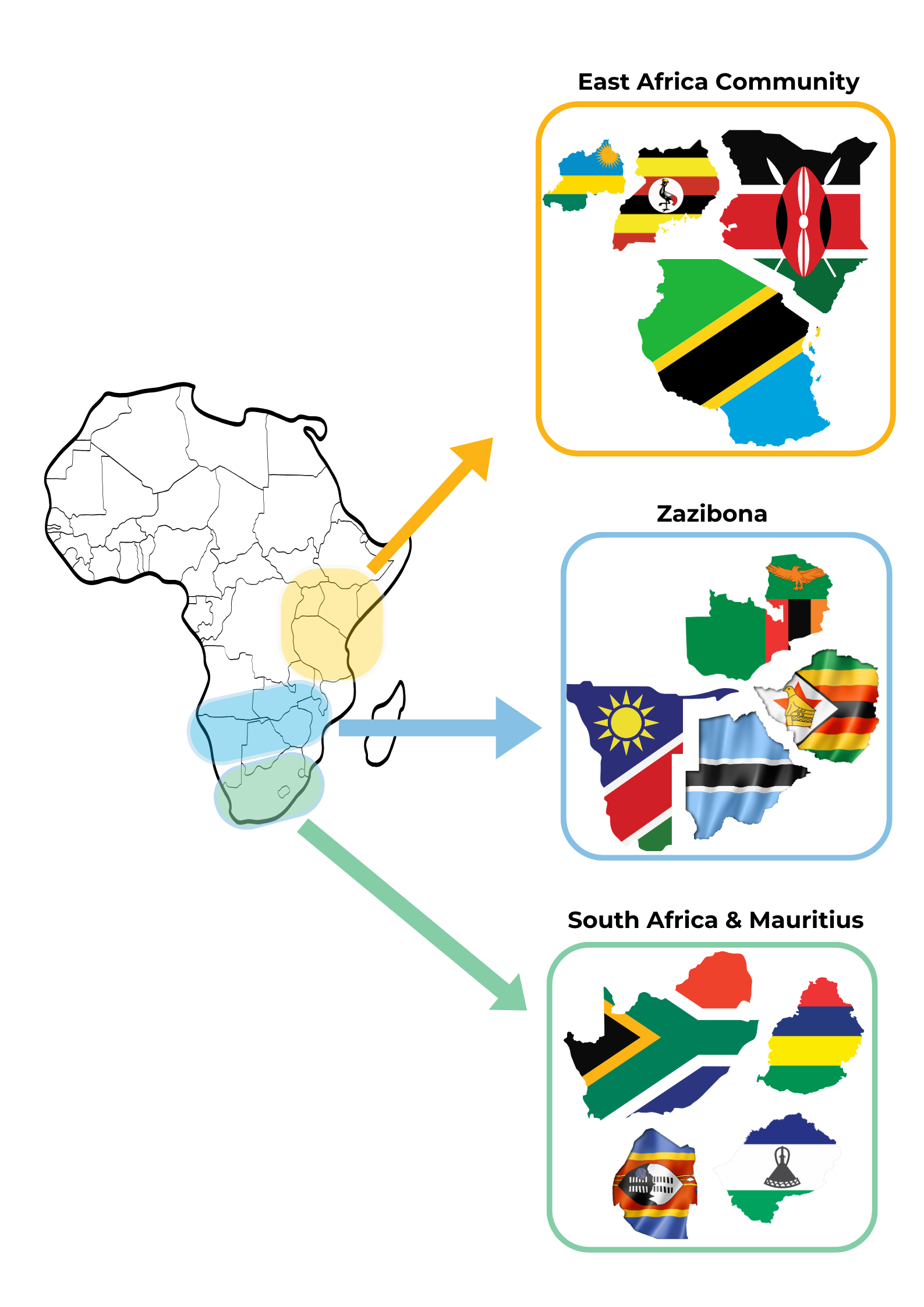
African Footprint
East Africa Community Medicines Regulatory Harmonization Programme (EAC-MRH) The East Africa Community Medicines Regulatory Harmonization Programme is a regional programme that was launched on 30th March 2012 by the EAC Council of Ministers in Arusha, United Republic of Tanzania. The goal of the programme is to facilitate access to safe, efficacious and quality essential medicines, vaccines and medical devices for treatment, management and diagnosis of conditions of public health importance. The programme does this through harmonization of regulatory requirements, guidelines, standards and tools for the EAC National Medicines Regulatory Authorities (NMRA). The countries included in this program is
Zazibona ZAZIBONA is a collaborative medicines registration initiative in Southern Africa focusing on dossier assessments and good manufacturing practice (cGMP) inspections. ZAZIBONA was founded in October 2013 by four countries Zambia, Zimbabwe, Botswana and Namibia with the support of WHO prequalification and the Southern Africa Regional Program on Access to Medicines (SARPAM). It currently includes the following active members:
South Africa and Mauritius The South African Health Products Regulatory Authority (SAHPRA) is the organisation in charge of regulating the use of all Health Products in South Africa. Mauritius regulates health products through a variety of acts and regulations, including the Public Health Act, the Food Standards Agency Act, and the Pharmacy Act. Lesotho's medicines and medical devices are regulated by the Lesotho Medicines and Medical Devices Control Authority. The country also relies on the South African Health Products Regulatory Authority (SAHPRA) and the World Health Organization (WHO) for regulatory approval. |
Licences & Certificates
Oryx Life Sciences provides a platform for the commercialisation of Healthcare Producs and ensure regulatory compliance of our Biopharmaceutical, Pharmaceutical and Neutraceutial clients.
SAHPRA compliant, GMP compliant, PICs member.



