OUR SERVICES
What we offer

Registration & Commercialisation of Allopathic Medicine
Registration of Commercialisation of Complementary Medicine

Registration of Commercialisation of Medical Devices and IVDs
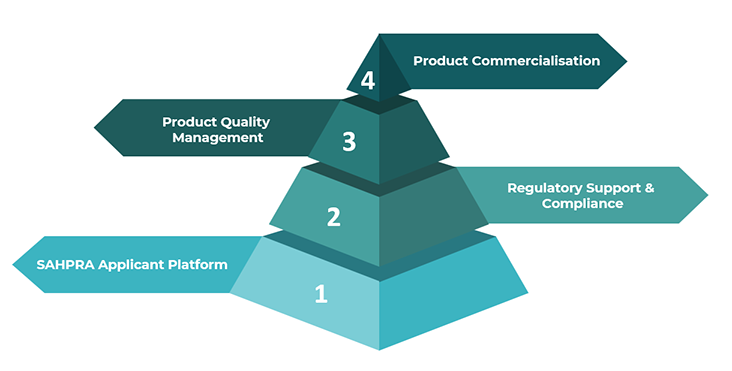
1. SAHPRA Applicant Platform
Applicant / Marketing Authorisation Holder Service enabling Marketing & Distribution Partnerships and Product In-Licensing Agreements:
SAHPRA Manufacturer, Importer and Exporter License
SAPC Manufacturing Pharmacy License DoH Premises License
PICs aligned Quality Management System
GMP compliant
ISO-13485 compliant
SAPC registered and recorded Responsible Pharmacist
2. Regulatory Support & Compliance
ICH-Aligned eCDT Dossier complilation, submission, registration
Allopathic (Category A) Human Medicines
Allopathic (Category C) Veterinary Medicines
Complemetary (Category D) Medicines
- Human
- Veterinary
Product Life Cycle Management, Registration & Listing of Medical Devices and IVDs
Medical Devices
In vitro diagnostics
Product Regulatory Support & Compliance
Adverse Event Reporting:
Robust pharmacovigilance systems to ensure patient safety
Labeling and Packaging
Comply with regulatory requirements for product labeling, packaging, and patient information.
3. Product Quality Management
Product Quality Management
Analytical testing
Batch Release
Product release
Deviations
Out-of–trends
Product reviews and trending
QMS maintenance
Product Out-of-specifications
Product complaint management
Product recalls
Change control
APQR
Pharmacovigilance
Facility Audits
4. Product Commercialisation
Product Business Case Development
Understand the Market through Market Research
Competitor Analysis
Stakeholder Analysis (healthcare providers, patients, payers, and distributors).
Patient Flow mapping
Sales Projections
Effective Marketing and Communication Strategy
Branding: Develop a strong brand identity and messaging
Multi-Channel Marketing
Key Opinion Leader (KOL) Profiling and Engagement
Patient Education
Medical Affairs Support
Phase IV Research
Marketing Material Approval
Sales Force Readiness
Sales Force employment
Training: Training on product, competitive landscape, and customer needs.
Territory Planning: Optimize sales territories to maximize reach and efficiency
Product Launch Management
Monitor launch performance throuogh sales, market share, and customer feedback.
Strategy adjustment based on real-time data insights
Monitor
Pricing and Reimbursement Strategy
Market-Based Pricing
Reimbursement Negotiations
Stakeholder / Account Management
Supply Chain Management
Stakeholder Management
Build and maintain relationships with prescribers
Patient awareness campaigns and support programs.
Payers and Policymakers
Risk Management
Legal and Ethical Considerations Intellectual Property Protection: Secure patents and trademarks to protect the product
Compliance: Ensure all promotional activities adhere to legal and ethical standards
Transparency: Maintain transparency in clinical data and marketing communications
Continuous Improvement
Adapt strategies based on market response and emerging trends
COMING SOON -
All products will soon be available in our online store - https://apotek.co.za
